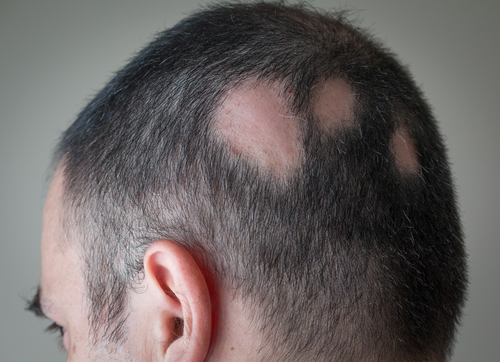
Novel use of an old systemic: Cyclosporine for Alopecia Areata
Alopecia areata (AA) is considered the most common autoimmune disease in humans with an estimated prevalence of 1 in 1000. Patients who have alopecia areata may present with acute or chronic patches of nonscarring hair loss. The hair loss of AA can range from a single patch to full body hair loss and can have an unpredictable course. While the exact etiology of alopecia areata is likely multifocal, AA is a T-cell–mediated autoimmune disease of the hair follicle. It is recommended to screen adults for thyroid disease who present with AA.
Optimal treatment of patients with AA remains elusive. First line treatment regimens include treatments with topical and intralesional steroids. Oral corticosteroids have been used for extensive or refractory disease. There is a need to examine additional treatment modalities for patients who suffer from AA.
A study published in this month’s JAAD investigates the use of cyclosporine for the treatment of moderate to severe alopecia areata. This was a double blinded placebo-controlled trial in adult patients. Study participants received a placebo or cyclosporine 4mg/kg/d for three months in a twice daily dosing. Many of the participants in the study had long standing, treatment resistant extensive disease. Alopecia was graded using the Severity of Alopecia Tool (SALT). Response was defined as 50% improvement of SALT scores. In this study, the participants who received cyclosporine in this study achieved a 31.3% response rate of their alopecia at 3 months compared to a 6. 3% response rate of the placebo group. It is important to weigh the adverse effects of cyclosporine including nephrotoxicity, hypertension and hyperlipidemia with the benefits of treatment. This study was limited by its small sample size and duration of study.
Byline: Sarah B. W. Patton, MSHS, PA-C
Posted September 18, 2019
Source: Lai VWY, Chen G, Gin D, Sinclair R. Cyclosporine for moderate-to-severe alopecia areata: a double-blind, randomized, placebo-controlled clinical trial of efficacy and safety. JAAD. 2019; 81(3): 694-701.







