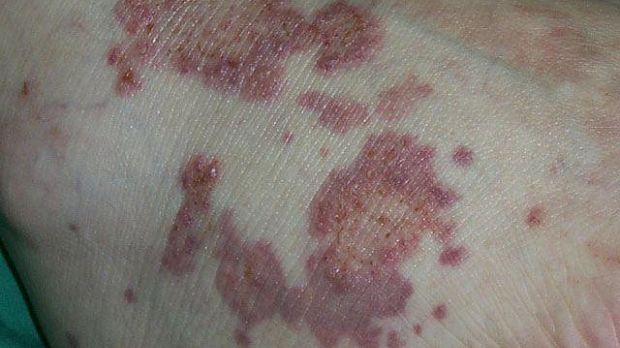
Thalidomide for Cutaneous Lupus Erythematosus: Which Patients Benefit?
Thalidomide shows promise for treating cutaneous lupus erythematosus (CLE) when the first-line treatment has failed. However, thalidomide has its drawbacks, including high cost and high teratogenicity. Clinical benefits must be weighed against potential serious adverse events such as peripheral neuropathy that does not resolve after end of treatment and thromboembolic events.
A recent systematic review and meta-analysis sought to assess the overall rate of response to thalidomide in patients with CLE, to compare rates of response to thalidomide between CLE subtypes, and to assess the rate of thalidomide withdrawal related to adverse events and the prevalence of severe adverse events, particularly peripheral neuropathy and thromboembolic events.
The authors found 21 papers in published and gray literature that met the study criteria and encompassed a total of 548 patients. On the basis of that sample, the results showed that thalidomide is associated with an excellent overall rate of cutaneous response of 90% in CLE, with complete response in more than 60% of cases. However, with that response rate came a number of adverse events. Peripheral neuropathies were frequent, with approximately 4% of those cases persisting after treatment was ended. There was also a pooled rate of 2% for thrombosis in the study population. About a quarter of the population withdrew from the treatment due to adverse events.
The authors conclude that although thalidomide shows excellent efficacy for CLE, this impressive result needs to be balanced against severe adverse events. They suggest that use of thalidomide should therefore be limited to patients with severe, refractory CLE or who are at high risk for severe scarring.
Byline: Martha L. Sikes, MS, RPh, PA-C
Posted: February 12, 2018
Source: JAAD
Adapted from the original article.
[Image: DermNet New Zealand]







